Before the advent of cultured meat, cell culture was primarily used in biomedical research, development, and production. Here, its main application was as a research tool, for human tissue engineering in regenerative medicine, and for the biotechnological production of biopharmaceuticals. Biotechnological production covers a broad range of approaches, from the fermentation of bacteria and fungi – e.g., for production of low-molecular weight therapeutics such as antibiotics or immunosuppressants – to the cultivation of genetically modified mammalian cell lines for the production of recombinant therapeutic proteins. Some of the bioreactors used in these processes have a capacity of more than 20,000 liters.
Upstream and downstream process
Upstream and downstream process
The manufacturing of a biopharmaceutical can be separated into a production, or “upstream process”, and a purification, or “downstream process”. During upstream processes, a production strain is usually generated by inoculation (artificially inducting immunity against various infectious diseases) from a master cell bank, followed by successive cultivation and scale-up from small culture flasks and laboratory-scale fermenters, up to production reactors at industrial scale.
Once the desired biopharmaceutical protein is produced in a sufficient amount, the downstream processes are applied in order to obtain the desired biopharmaceutical product. The tools and processes used for the production of cell-based meat are similar to the processes described above. However, in this case, the cells themselves represent the final product, and the major challenge lies in the upstream process. Because of the larger scale of cell production envisaged for cultured meat, and the necessity for competitive pricing compared to conventionally produced food, a highly efficient and economical production process is needed. Product safety requirements for food are less stringent than those for pharmaceutical drugs and cell therapies.
The elements of ‘meat’
A comprehensive definition of “meat” can be found in the European meat hygiene law (Regulation (EC) No 853/2004). “Meat is, in principle, considered as skeletal muscle deriving from specified animal species, which may include specified edible offal and blood" [23]. More generally, from a consumer perspective, meat can be defined as skeletal muscle with connective tissue and adherent fat. The focus of this chapter is therefore primarily on muscle cell culture. To explain how muscle cells can be produced in vitro, this section will address four major aspects: the cells, the culture medium, the scaffolds, and the process for meat production.
Installing bioreactors
The industry is still working to refine its terminology. In the future, a factory that creates cultivated meat may be called a “carnery” (from the Latin word for “meat”, carnis), a “meat factory”, a “meat brewery” or something else. In any case, it will contain one or more tanks for the large-scale production of in-vitro meat similar to brewing vats. Currently, these are known as “bioreactors”, though a more neutral and market-friendly term such as “growth tanks” may be preferable.
Cells
Cells
Cattle, pork, chicken, fish, and other animal species are used for meat cell culture. Primary cell cultures are obtained by collecting cells directly from an animal. These cells have a finite lifespan and limited capacity to divide. They are sensitive and require specific nutrients and growth factors in the medium. Their key advantage is that the morphology and physiological characteristics of primary cell cultures are close to those of the original cells. Therefore, cultured meat is mostly based on primary muscle cell culture.
Some cells can become immortal through a process called transformation, which can be experimentally induced or can occur spontaneously. Immortal cell lines can be quite different from the original cells. If a cell is initially induced using genetic engineering methods, the cell line may eventually have to be declared as genetically modified. Immortalized cell lines could potentially decrease the dependency on freshly obtained primary cells and increase the speed of differentiation and proliferation [24]. However, it remains to be seen whether they will be accepted by consumers.
Myogenesis: muscle formation
The process of muscle formation is called myogenesis. Skeletal muscle formation happens in several steps and includes embryonic and adult skeletal myogenesis. After birth, muscles can only grow by training (hypertrophy), but not by division of cells (hyperplasia) [25]. One exception is muscle regeneration in adults after trauma [26]. This is triggered by one type of tissue-specific stem cell, the so-called myosatellite cells. In skeletal muscle tissue, myosatellite cells are located outside of the muscle fibers and are usually in a dormant, non-dividing stage. In case of physical trauma, these myosatellite cells are triggered and undergo myogenesis.
In cell-based meat production, the aim is to reproduce this process of naturally occurring myogenesis in skeletal muscle cell culture (see illustration below). As an organism ages, the regenerative capacity of its myosatellite cells decreases, which is why they are preferentially collected from fetuses or neonate animals. Upon stimulation, the stem cells can develop into myoblasts, which can proliferate if enough growth factor is present. When the growth factor runs out, myoblasts stop dividing and undergo differentiation into multinucleated myotubes. In a subsequent stage of differentiation, myotubes align with each other and form myofibrils, resulting in a meat-like structure.
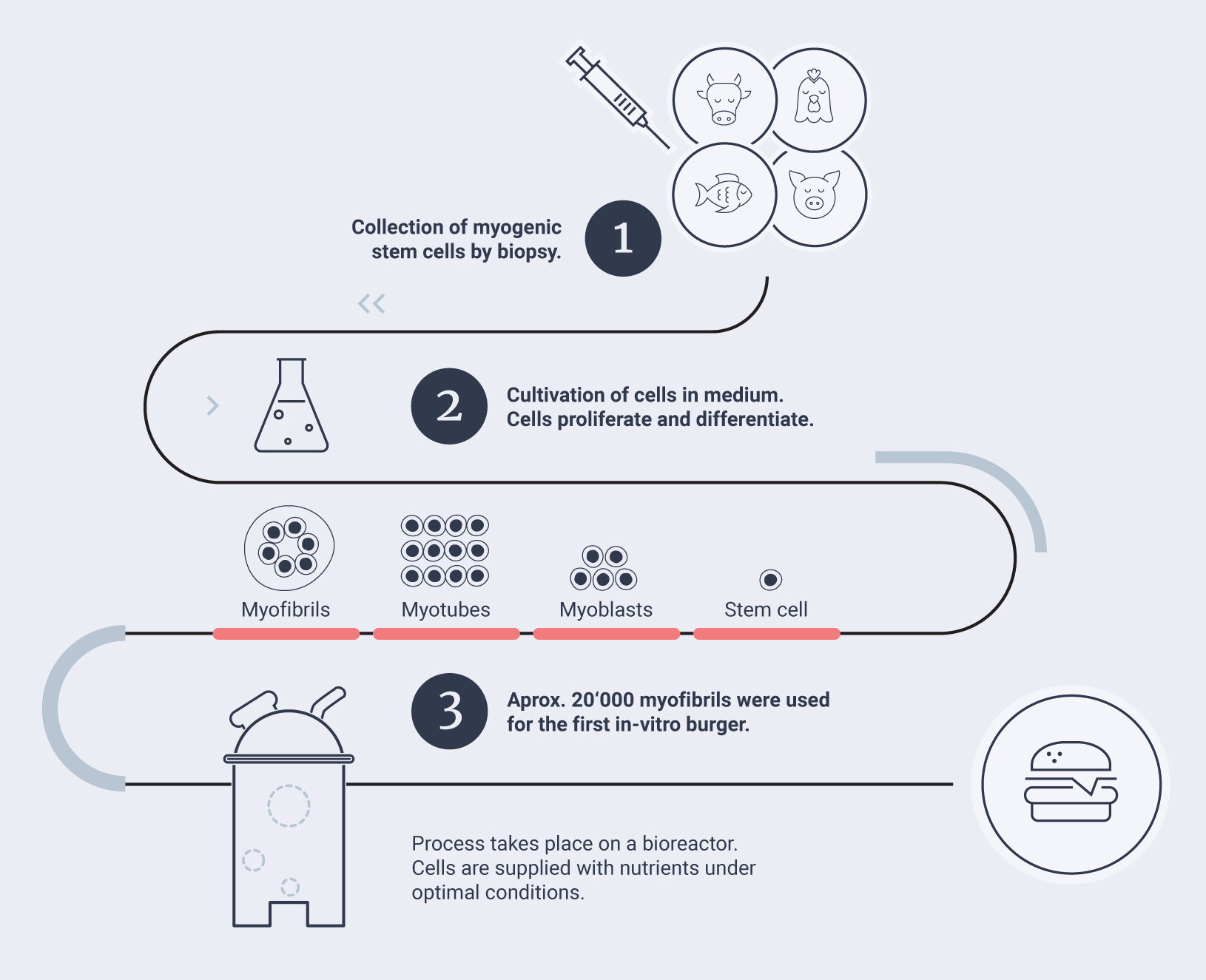
Evolved structures
The most challenging step in terms of myocyte culture is the myofibril formation, which requires a scaffold (see Chapter 4.4). When collecting cells to start a culture, not only the species, but potentially also specific breeds of a species or even the individual animal might be important. It can be assumed that different breeds of cattle can vary in terms of the proliferation and differentiation of their myogenic stem cells and myoblasts when used in cell culture.
This is due to the legacy of hundreds of years of conventional breeding to increase the amount and quality of muscles, or the proportion of fat in the muscles. For example, the double-muscled Belgian White Blue and Piedmontese cattle breeds carry a natural mutation, which causes a gene mutation in their myostatin gene. This gene codes for the protein that normally inhibits muscle development. The gene mutation results in an accelerated growth of lean muscle. Cells from this breed were recently assessed in cell culture [27].
The company Just, Inc. plans to develop cultured Wagyu beef using cells from Toriyama prize cows [28]. In higher animals, the liver is the only visceral organ with the capacity to self-regenerate by stem cell differentiation. The cellular agriculture company Integriculture develops liver cell cultures to produce foie gras as one of its products.
Culture meadia
Culture meadia
Growth and proliferation of cells in in-vitro culture requires media that contain all necessary nutrients. The actual constituents of various cultured meat media are usually unknown, since they are trade secrets that are closely guarded as intellectual property (IP). Beside salts, amino acids, carbohydrates, vitamins, peptides, and proteins, cell culture media often contain bovine calf serum (BCS). The cells must be supplied with necessary hormones, growth factors, and less narrowly defined components. Bovine calf serum can also be derived from fetuses; in this case, it is called fetal calf serum (FCS). So far, BCS is still a common key supplement for the cultivation of mammalian cells [29].
Currently, BCS is derived from conventional farming and slaughtered animals. As one source explains it, “Bovine serum is generated as by-product of the meat industry. It is taken at the time of slaughter, from adult cattle, calves, very young calves or, when cows that are slaughtered are subsequently found to be pregnant, from bovine fetuses” [29]. Recently, Multus Media has developed Proliferum M[88], a nutritious serum substitute that is safe to use for food design. The company currently prices Proliferum at $100USD/L but is working on reducing that price to $1USD/L[89] to make it more accessible for large scale production.
Serum-free media
Significant efforts have been undertaken to procure serum-free cell culture media. This research was initially motivated by the cell-therapy industry in order to reduce health risks for patients receiving cell therapies. The goal was to avoid potential complications from adventitious contaminating agents, such as prion or viral diseases or other disorders. Many recipes for serum-free media have been published [30];[31]. The key question is, however, how effective serum-free media are in the large-scale production of in-vitro meat.
A recipe for a serum-free medium to be used in the cultivation of primary bovine myoblasts has recently been published by the University of Maastricht in the Netherlands [32]. Cells were collected from a cow, and myoblasts were subsequently cultured in different media. However, the researchers conclude “that serum-free media stimulate exponential cell expansion, albeit not to the extent of the current growth medium containing up to 30% serum. Further research is needed to investigate whether prolonged cell culture or an adaptation period could further increase cell proliferation.”
Secret recipes
Cultured meat companies also work on serum-free media and develop their own secret recipes. Mosa Meat has announced a growth medium without serum, adding that “[…] scientists in our medium team have been working to completely remove FBS and all other animal components from our growth media. There were no equivalents that worked as well, so this has been no small feat. And we’re really happy to be able to say that we’ve succeeded in developing our own entirely animal component-free growth media, which are performing really well” [33]. Furthermore, Integriculture Inc. (2019) has stated that it is developing an automated bioreactor system to produce artificial serum.
Due to the effort required to produce sterile BCS, the price for this medium component is relatively high. Currently, one liter of BSC can cost €133 [34], reflecting the cost of a highly qualified product used at research scale, while lower-cost BSC might be used at production scale. Usually, 10 to 20 percent of the BCS in the medium is used [24]. Even assuming that the price per liter is considerably lower for the larger lots needed at production scale, it is obvious that BCS is one of the main cost drivers for culture media. Van der Weele and Tramper (2014) estimate that “[a] price of €1 per liter for growth medium would bring the price of minced-meat type of product within the price range of conventional minced meat, but this is when only the price of medium is considered”.
Cost calculations
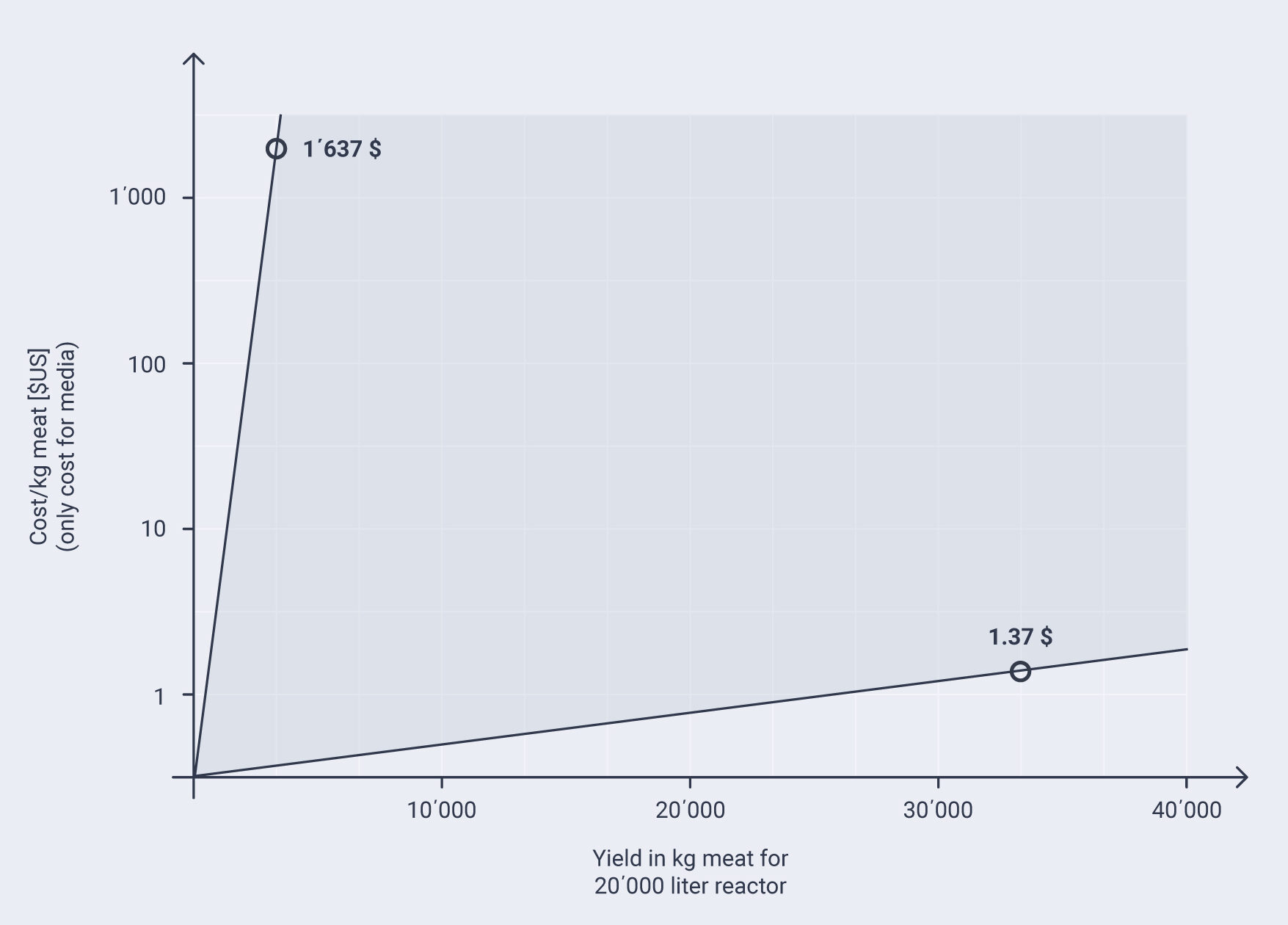
A recent report by the Good Food Institute – GFI [35] provides a hypothetical assessment of medium costs for a fed-batch of 20’000 liters. Fed-batch means the medium remains in the reactor until the end of the run. Specht bases her calculation on the generally used serum-free medium Essential 8TM [36]. The medium requires hormones and growth factors representing over 99 percent of the cost total costs. Based on a variety of assumptions, the author models cost between US$0.24 and US$40.94 per liter of medium and costs between US$1.37 and US$1,637.60 for the medium per kg of in-vitro meat produced (see chart). The author further concludes that “[…] the scale of production for cultivated meat will be orders of magnitude larger than current animal cell applications, so there remains significant potential from achieving true economies of scale.” In addition to ethical and economic aspects, there are also feasibility and product safety arguments in favour of serum-free media.
As of now, it is unclear whether the current procedures for obtaining BCS and the resulting volumes will be sufficient for massive scale-up. Moreover, the producers of cultured meat will have to demonstrate product safety. When cultured cells are harvested, media components can hardly be removed completely from the end product, and any animal-derived component could potentially contain so-called adventitious contaminating agents. Reducing the animal-derived components in the medium would also lower this risk.
The typical taste and color of conventionally produced meat is a combination of multiple factors such as animal species, breed, age, food, conditions under which the animal was raised, maturation processes after slaughtering, the proportion of fat, and last but not least the heme proteins in the blood. Extracellular heme protein myoglobin in the culture medium improves bovine myosatellite cell proliferation and the color of cell-based meat [37].
Antibiotics
Aseptic conditions are a key requirement for cell culture to avoid contaminations with bacteria and other microorganisms. The use of antibiotics in cell cultures is widely practiced in research, in particular for long-term culture. An encouraging finding is that antibiotics are not necessarily required in the culture of bovine myoblasts and that elimination of antibiotics even resulted in higher cell growth. In the experiments where the myoblasts were allowed to proliferate for up to six days, the researchers did not observe any contamination [32]. However, it remains to be seen whether this finding can be fully translated from the research laboratory to production scale.
If the assumptions made by Specht (2020) in modeling the production process are correct, cells would be in culture for 40 to 74 days. It is obvious that maintaining sterile conditions throughout such a long production process will be one of the fundamental challenges. If antibiotics are used, the thresholds applicable for food would need to be observed for the cell-based meat product. The same applies to undesired metabolic by-products that would originate from the production process itself (some of the challenges of using antibiotics are presented in Chapter 6).
Stem cells are able to develop into all the different cell types that make up the human body. Most of the insights into how stem cells work is derived from regenerative medicine, where healthy cells are used to replace diseased cells. Embryonic stem cells have the potential to differentiate into basically any tissue under appropriate conditions. Adult stem cells can only develop into a pre-determined type of tissue. Myosatellite stem cells, which are often used for meat culture, differentiate into muscle tissue.
Scaffolds
Scaffolds
A tissue is defined as an ensemble of similar cells (including their extracellular matrix) that carry out a specific function. In tissues, the cells are always in direct contact with neighboring cells and the extracellular matrix. The functional grouping of multiple different tissues forms organs.
Muscle cells are anchorage-dependent and have the capacity to contract spontaneously. Anchorage dependence means that the cells only proliferate when they are attached to a surface and to each other. Therefore, a suitable structure for cells to grow on, a so-called scaffold, must include a large surface for attachment and growth, be flexible to allow for contraction, facilitate diffusion of the culture medium into the cells, and must either be easy to separate from the cultured meat or be edible.
Scaffold materials
An ideal scaffold would also be cheap and available in sufficient amounts for scale-up. Many different materials appear to be suitable for scaffolds, including sponges, membranes, fibers, and beads. Potential materials could be made from plants, mushrooms, chemicals such as chitin, or from animal-derived materials like collagen. Most of the successfully synthetized bio-artificial muscle has been grown on collagen and achieving tissue contraction with synthetic biomaterials has been challenging. Since scaffolds are key to culture cells, they are the focus of both active research and intellectual property protection [38], [39], [40],[41].
Tofu and soybean proteins have also been assessed for their suitability as scaffolds for tissue engineering (see illustration). Very recently, researchers from the Technion-Israel Institute of Technology and Aleph Farms have claimed to have achieved a breakthrough using textured soy protein as an edible scaffold for bovine muscle tissue engineering. Bovine satellite cells were cultivated in the presence of myogenic-related growth factors, leading to an elevated myogenesis, either alone or in co-culture with other cell types [42].
Scaffold-free solution
An alternative to the use of scaffolds is to modify cells in order to induce anchorage-independence and thus independence from scaffolds. Memphis Meats claims that inhibiting the “hippo pathway”, which controls the size of animal organs through regulation of cell proliferation, cell death (apoptosis), and stem cell self-renewal, induces anchorage independence of cells and increases culture yields (see patent overview, WO2018208628A1).
Future Meat has filed patents on the use of a spontaneously immortalized chicken fibroblast line and a closed-loop perfusion bioreactor system in an attempt to trigger adipogenesis and myogenesis from these fibroblasts (see patent overview, WO2018011805A9).
Processes
Processes
In living animals, endothelial tissue forms blood vessels through which oxygen and nutrients are supplied and metabolic waste products are removed. The tissues grow in an environment with a specific level of CO2 gas pressure and at body temperature. However, when cultivated in vitro, cells grow on surfaces in layers up to a thickness of approximately 0.1 to 0.2 mm [43]. Their viability is highly dependent on the process of physicochemical diffusion. The production processes need to account for all these requirements.
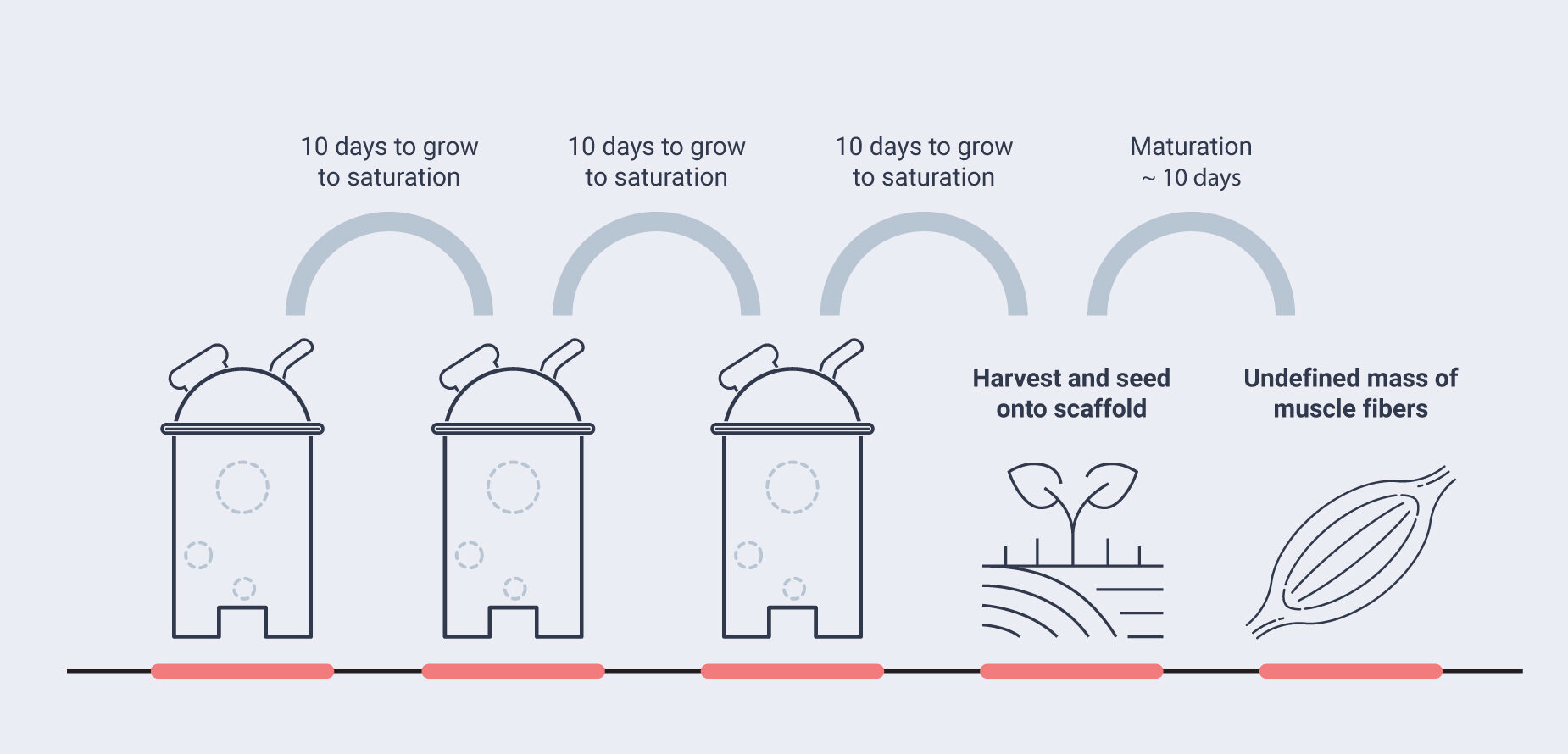
Traditional fed-batch bioreactor systems often consist of vessels with a volume of 3,000 to 20,000 liters. Cells are cultured in batches that typically run from one to three weeks until the media nutrients have been consumed and waste products accumulate. In perfusion bioreactors, however, the cells are fed with fresh media and spent media and removed on a continuous basis. This allows cells to be kept in culture over much longer periods. In order to avoid losing cells through the perfusion or harvesting process, cells are kept in the bioreactor by different technologies, such as by growing on scaffolds, filtration, centrifugation, the specific geometry of the cultivation vessels, or by other means.
Patented technologies and processes
Very little reliable and detailed information is publicly available on actual meat culture cell production processes since companies seem to be selective in disclosing information.
However, to get an idea which type of IP has been claimed by in-vitro meat companies, we looked at examples from Aleph Farms, Empire Technology Development, Fork and Goode, Future Meat Technologies, Integriculture, Just, Memphis Meats, Modern Meadow, Mosa Meat, SuperMeat, and Wild Type (See Table below). In principle, patents can be divided into inventions related to biological cells and those concerning process engineering.
| Organization | Patent | Titel |
|---|---|---|
| Aleph Farms | WO2015008275A1 | Methods for large-scale generation of stem cells |
| Aleph Farms | WO2019016795A1 | Cultured Meat Compositions |
| Empire Technology Development | US20170253849A1 | Synthetic Meat |
| Fork and Goode | US20190376026A1 | Large scale cell culture system for making meat and associated products |
| Future Meat Technologies | WO2018011805A9 | Systems and methods for growing cells in vitro |
| Integriculture | JP6111510B1 | Growth induction system, growth induction control device, growth induction control method, and growth induction control program |
| Just Inc. | WO1999031222A1 | Industrial-scale production of meat from in-vitro cell cultures |
| Just Inc. | US6835390B1 | Method for producing tissue-engineered meat for consumption |
| Memphis Meats | WO2015066377A1 | Method for scalable skeletal muscle lineage specification and cultivation |
| Memphis Meats | WO2017124100A1 | Methods for extending the replicative capacity of somatic cells during an ex-vivo cultivation process |
| Memphis Meats | WO2018208628A1 | Compositions and methods for increasing the culture density of a cellular biomass within a cultivation infrastructure |
| Memphis Meats | WO2019014652A1 | Compositions and methods for increasing the efficiency of cell cultures used for food production |
| Modern Meadow | US8703216B2 | Engineered comestible meat |
| Modern Meadow | US20160227831A1 | Dried food products formed from cultured muscle cells |
| Modern Meadow | WO2014039938A1 | Spherical multicellular aggregates with endogenous extracellular matrix |
| Modern Meadow | WO2014110250A1 | Methods and devices for preparing and continuously printing multicellular cylinders onto biocompatible substrates |
| Modern Meadow | WO2015038988A1 | Edible and animal-product free microcarriers for engineered meat |
| Mosa Meat | US20190338232A1 | Apparatus and process for production of tissue from cells |
| Super Meat | WO2018189738A1 | Cultured meat-containing hybrid food |
| Wild Type | WO2018227016A1 | Ex vivo meat production |
The usefulness of these patents can be considered from various angles. For example, the patents from Just, Inc. enjoy broad coverage and could be very powerful if enforceable. Inventions that have the potential to significantly reduce the production costs might be very useful as well. Already in 1997, cell-based meat pioneer Willem van Eelen and colleagues submitted a patent for “Industrial scale production of meat from in vitro cell cultures” (patent no. WO1999031222A1). The list of patents provided in the table above is far from being fully comprehensive. Inventions that are made in related areas such as biopharmaceutical production, medical tissue engineering, or processing of food are likely to enhance cultured meat technology development as well, but these inventions are not obvious when searching for cell-based meat inventions.
The extent to which cell-based meat companies have actually developed their production processes based on these patents remains unclear. It is likely that a significant part of the technological know-how has not been published either in form of patents or in scientific journals and simply belongs to these companies as proprietary trade secrets.
Ensuring safe processes
For the downstream process after cell harvesting, foodborne pathogens may also play a role. Nontyphoidal Salmonella spp in meat cause many episodes of human illness every year [44]. At this stage, producers ensure aseptic conditions or could make use of established food preservatives such as sodium benzoate [45], always considering the legally accepted thresholds for food.
Processes such as those described above depend on deep expert knowledge and skills, which are currently concentrated at a very few sites. However, decentralized models can be conceptualized. Already in 2014, Van der Weele et al. postulated that every village may eventually have its own cultivated meat factory. Others suggest that at some point in the future, in-vitro meat bioreactors may even be a feature in every household. At the moment, though, it seems very unclear whether and when such concepts may become feasible and/or desirable.
Yield calculations
Specht (2020) has modelled hypothetical multi-step batch-fed production process schemes, from inoculum through seed train to a maximum reactor volume of 20,000 liters for the proliferation stage. The author also calculates the hypothetical total meat yield for a multi-harvest production run, and the overall length of the production run for two scenarios (50 percent and 90 percent harvest yields, respectively). This would hypothetically result in a meat yield ranging from 3,500 kg after a run time of 40 days to a yield of 31,850 kg after 74 days. As these numbers suggest, the modeled data demonstrate a huge spread, depending on the technical and cost assumptions for the actual process.
When describing cultured meat production, it is important to distinguish between unstructured and structured cultured meat. Unstructured meat is a mass of myofibers that is suited, e.g., for burger patties, but is unlike a traditionally produced meat cut that also contains fat, endothelial tissue, and collagen. Structured cultured meat products will be closer in texture to traditional meat cuts.
Extrusion, 3D-printing and co-cultivation
One possibility is to apply downstream processing steps such as re-formatting the cultured meat products and adding other components such as fat or heme proteins in order to obtain a better taste and color. For example, patents for extrusion and printing steps have been filed by Modern Meadow (see patent overview, WO2014110250A1).
Another concept is to co-cultivate different types of cells, e.g., myocytes and fat cells, in the same culture with the goal of creating a more meat-like structure. When co-cultivating cells, it seems more difficult to achieve optimal conditions for proliferation and differentiation of the cell types used in parallel.
Supertrends experts such as Nina Buffi predict that the development of unstructured meat products is less complex, and that these will be available to the customer earlier than structured products.
Takeaways
Takeaways
Technologies used for cultivating meat are rather similar to established processes in biopharmaceutical production and tissue engineering in regenerative medicine, with cultivated meat being mostly based on primary bovine myocyte cell culture. If immortal cell lines are used, the end-product may be classified as genetically modified food.
In extracting cells from animals, not only the species matters, but the breed may also be important with regard to the cells’ capacity to proliferate and differentiate. Growth factors, e.g., from bovine calf serum, are essential elements and the key cost driver in the culture medium, while scaffolds are important for an efficient cell growth and production process. Processing steps after cell harvest may also play a role in creating the final, meat-like product. The costs involved in cultured meat production are difficult to model, and developing cost-efficient production processes remains a central challenge.
References
[23] Orzechowski, A. 2015. Artificial meat? Feasible approach based on the experience from cell culture studies. February. Journal of Integrative Agriculture 14(2)
[24] Lautenschlaeger, R. and M. Upmann 2017. How meat is defined in the European Union and in Germany. Animal Frontiers 7(4):57–9.
[25] Grefte, S. et al. 2007. Skeletal muscle development and regeneration. Stem Cells and Development 16(5):857–68.
[26] Kolkmann, A.M. et al. 2020. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 72(1):111–20
[27] Just. https://www.ju.st/en-us/stories/meat (accessed April 2020)
[28] International Serum Industry Association. Frequently Asked Questions - Bovine Serum. https://www.serumindustry.org/documents/sera20110511_000.pdf. Accessed 19 March 2020.
[29] Brunner, D. et al. 2010. Serum-free cell culture: the serum-free media interactive online database. Alternatives to Animal Experimentation 27(1):53–62.
[30] Van der Valk, J. et al. 2010. Optimization of chemically defined cell culture media – replacing fetal bovine serum in mammalian in vitro methods. Toxicology In Vitro 24(4):1053–63.
[31] Kolkmann, A.M. et al. 2020. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 72(1):111–20.
[32] Mosa Meat 2019. Growth Medium without Fetal Bovine Serum (FBS). https://www.mosameat.com/blog/2019/11/15/mosa-meat-on-netflixs-explained. Press release, 19 November 2019. Accessed: 3 March 2020.
[33] Sigma-Aldrich catalogue (https://www.sigmaaldrich.com/catalog/product/sigma/12133c?lang=de®ion=DE&cm_sp=Insite--prodRecCold_xviews--prodRecCold10-5. Accessed 19 March 2020).
[34] Specht, L. An analysis of culture medium costs and production volumes for cultivated meat. The Good Food Institute report. Updated 9 February 2020.
[35] Chen, G. et al. 2011. Chemically defined conditions for human iPSC derivation and culture. Nature Methods 8(5):424–9.
[36] Simsa, R. et al. 2019. Extracellular Heme Proteins Influence Bovine Myosatellite Cell Proliferation and the Color of Cell-Based Meat. Foods 8(10):521.
[37] Enrione, J. et al. 2017. Edible Scaffolds Based on Non-Mammalian Biopolymers for Myoblast Growth. Materials 10(12). pii: E1404. doi: 10.3390/ma10121404.
[38] MacQueen, L.A. et al. 2019. Muscle tissue engineering in fibrous gelatin: implications for meat analogs. NPJ Science of Food 21(3):20.
[39] Orellana N. et al. 2020. A New Edible Film to Produce In Vitro Meat. Foods 9(2):185. pii: E185. doi:10.3390/foods9020185.
[40] Marga F. et al. 2015. Edible and animal-product-free microcarriers for engineered meat. Worldwide patent no. WO2015038988A1, 19 March 2015.
[41] Ben-Arye, T. et al. 2020. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nature Food 1:210–20.
[42] Datar, I. and M. Betti 2010. Possibilities for an in vitro meat production system, January. Innovative Food Science & Emerging Technologies. 11,13-22
[43] Scallan, E. et al. 2011. Foodborne illness acquired in the United States – major pathogens. Emerging Infectious Diseases 17(1):7–15. Also available at CDC website: https://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html. Accessed: 26 March 2020.
[44] Piper, J.D. and P.W. Piper 2017. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Comprehensive Reviews in Food Science and Food Safety 16(5):868–80.
[45] Van der Weele, C. and C. Driessen 2013. Emerging Profiles for Cultured Meat; Ethics through and as Design. Animals 3(3):647–62. doi:10.3390/ani3030647.
[88] https://www.multus.media/products
[89] Cell Based Tech Weekly https://cellbasedtech.com/2020/07/cell-based-tech-weekly-pre-order-multus-media-proliferum-m-mosa-meat-gets-5-7m-from-bell-foods-group-berkeley-lights-ipo-soars
