Background
The term “proteostasis” denotes the multiplicity of biological pathways in the cell that interact, but also compete with one another in shaping and controlling the behavior of proteins both within and outside of the cell. This includes mechanisms for folding, stabilizing, and degrading proteins in an orderly and coordinated manner. Proteostasis has been shown to be affected by the aging process, and the degradation or loss of the cell’s ability to maintain the balance of these diverse pathways is associated with age-related diseases.
Unlike inanimate mechanical objects, in which minor damage can slowly accumulate until the object loses its functionality (think of an old car), cells are dynamic, living things. Their molecular structures are constantly being made, damaged, broken down, and remade. While damaged molecules do indeed tend to accumulate in our cells as we age, this is not necessarily because they just sit there taking damage. Rather, the issue has more to do with a decrease in turnover.
Damage is not a big problem if parts are constantly being renewed. If a car is constantly being overhauled by a mechanic and its parts replaced, its performance will not necessarily degrade over time. As we have previously seen, taking damage can even be a good thing in living organisms (see the section on hormesis). Certain types of damage tell the cells to ramp up their defences and repair mechanisms – physical exercise being just one example of many such stimuli.
Hormesis works because cells experience a constant flux of molecules. Proteins are not like car parts that stay around forever; rather, they are made and often quickly broken down again. Thus, when we observe accumulations of damaged molecules in old cells, this is not necessarily because they are suffering more damage, but might also be due to the cellular “garbage collectors” and “repairmen” not doing their job properly anymore.
One of the chief recycling systems in the cells is autophagy. Japanese cell biologist Yoshinori Ohsumi won the 2016 Nobel Prize for discovering and describing its mechanisms.[131] In essence, autophagy serves as a cellular garbage collection mechanism. Small structures in the cells recycle their contents by enclosing old and damaged molecules as well as debris and taking them away to be broken down. Subsequently, many of the spare parts (which are not broken themselves) can be reused to make new molecules.[132]
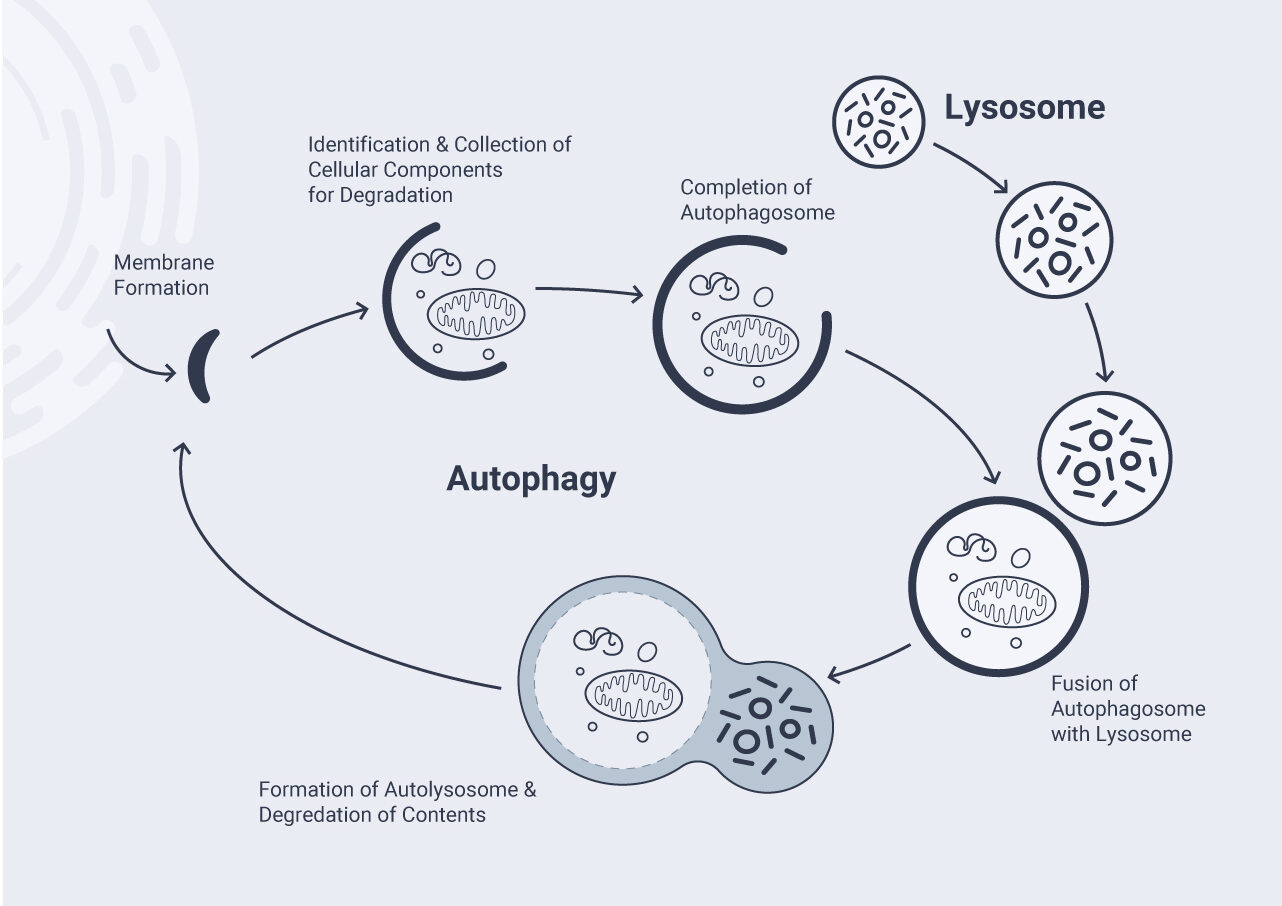
Unfortunately, this process breaks down as we age.[133] Old cells have less autophagy than young cells. This means it takes longer for their molecules to be removed. That phenomenon, coupled with the fact that old cells also experience more damage, makes for a deadly cocktail. There is reason to believe this process plays a key role in aging. Mice with enhanced autophagy are healthier,[134] and mice with faulty autophagy age rapidly.[135]
Autophagy has been implicated in multiple other aging hallmarks. For instance: Rapamycin extends the lifespan of model organisms, but it only works if their autophagy is functioning properly.[136] The process of debris removal is also involved in the response to calorie restriction and fasting. When the body does not receive sufficient building blocks from the outside (because it is starved), it will try to find them inside itself. To do so, it boosts autophagy to break down old and damaged molecules in order to reuse their components.
Besides autophagy, our cells have multiple other systems to carry out quality control on our proteins and ensure the breakdown of damaged molecules. Cells make proteins called chaperones, which help other proteins fold correctly (so they do not end up as cellular junk). When the cell is somehow stressed, this production is boosted but it becomes impaired in aging.[137] But if it is artificially boosted in model organisms, they live longer.[138]
Finally, another cellular “garbage collection system” – the ubiquitin-proteosome system – has also been implicated in aging. Its activity also seems to decrease drastically with age.[139] Activating it extends the lifespan of yeast, and makes human cells appear younger.[140]
Challenges
While autophagy and other quality control pathways are heavily involved in aging, they can also create vulnerability to cancer. Autophagy is a tool of the cell and[141] for tumor cells[142][143] by allowing them to override the suicide switches that are built into all our cells to prevent them from becoming cancerous.[144]
Road to Success
Researchers have found that fasting, calorie restriction, and exercise can boost autophagy.[145][146][147] The polyamine spermidine has shown promising results in stimulating autophagy in mice.[148]
Just as with telomere-based interventions, autophagy must be handled carefully due to the risk of cancer. Autophagy enhancement is a lot less risky than telomere-based interventions and has a higher likelihood of success, but cancer is an ever-present danger when biological organisms are manipulated. This is especially true in anti-aging interventions, where we walk a difficult tightrope in that we want our cells to survive better without becoming cancerous.
Once again, anti-aging treatments would be helped greatly by progress in cancer therapies. As we have previously noted, this would only increase the average life expectancy moderately, but it would also allow us to experiment more aggressively with certain life-extension interventions.
Companies
Selphagy Therapeutics (Life Biosciences)[149]
Website http://www.lifebiosciences.com
Industry Biotechnology
Company size 51-200 employees
Headquarters Boston, Massachusetts
Type Privately Held
Founded 2017
Selphagy Therapeutics was founded by Ana Maria Cuervo and Evis Gavathiotis. It is a daughter company of Life Biosciences (which is involved in rejuvenation companies that target multiple hallmarks of aging). The company aims to identify new autophagy-activating compounds that can be used for life extension. Specifically, Selphagy Therapeutics plans to focus on what is called chaperone-mediated autophagy – a pathway implicated in neurodegeneration and metabolic disorders, among other diseases. The company aims to boost this autophagic pathway using a retinoic acid receptor alpha inhibitor.
Samsara Therapeutics[150]
Website https://www.samsaratherapeutics.com/
Industry Biotechnology
Company size 11-50 employees
Type Privately Held
Founded 2018
Samsara Therapeutics was founded by a team of experts in the biology of aging, including Frank Madeo, who discovered the autophagy booster spermidine. The company aims to develop medicines to extend healthy lifespans, including by testing the flavonoid 4,4’-dimethoxychalcone, which has been shown to promote autophagy-dependent longevity in model organisms.[151]
Casma Therapeutics[152]
Website http://casmatx.com
Industry Biotechnology
Company size 11-50 employees
Headquarters Cambridge, Massachusetts
Type Privately Held
Founded 2018
Casma Therapeutics was founded by four autophagy experts including the late Beth Levine. The company’s approach is based on boosting autophagy in a number of diseases such as muscle disorders, liver disease, inflammatory disorders, and neurodegeneration. Their lead program is based on TRPML1 agonists for Gaucher and Parkinson’s disease. With its expertise, Casma Therapeutics is well positioned to help bring autophagy-based drugs to the market. These drugs can later be examined for their effects on lifespan, where the company’s autophagy expertise will also be very valuable.
Neuropore Therapies[153]
Website http://www.neuropore.com
Industry Pharmaceuticals
Company size 11-50 employees
Headquarters San Diego, CA
Type Privately Held
Founded 2008
Neuropore Therapies is a pharmaceutical company that focuses on developing treatments for neurodegenerative diseases. It does so by targeting the triad of increased inflammation, increased aggregation, and decreased clearance of “garbage” in the cell (due to a decrease in autophagy). Neuropore Therapies are targeting three subtypes of autophagy: macroautophagy (usually simply called autophagy), chaperone-mediated autophagy (which, as the name implies, is assisted by chaperone proteins), and mitophagy (the specific autophagy of mitochondria, as previously described).
Phoremost[154]
Website http://www.phoremost.com
Industry Biotechnology
Company size 11-50 employees
Type Privately Held
Founded 2014
Phoremost is a pharmaceutical company that has developed the drug target identification platform SITESEEKER. It uses this platform to discover new drugs and also collaborates with other pharmaceutical and biotechnology companies. Its main areas of activity include oncology (cancer), neurodegenerative diseases (including targeting senescence and autophagy), and targeted protein degradation. Its current pipeline includes three compounds named PM-008, PM-009, and PM-010, which the company is currently researching for their ability to modulate autophagy and senescence.
References
[131] https://www.nobelprize.org/prizes/medicine/2016/press-release/
[132] Mizushima, N. 2007. Autophagy: process and function. Genes & Development 21:2861-73.
[133] Rubinsztein, D.C. et al. 2011. Autophagy and aging. Cell 146(5):682-95.
[134] Zhang, C. and A.M. Cuervo 2008. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nature Medicine 14(9): 959-65.
[135] Rubinsztein et al. 2011.
[136] Bjedov et al. 2010.
[137] Calderwood, S.K. et al. 2009. The shock of aging: molecular chaperones and the heat shock response in longevity and aging: A mini-review. Gerontology 55(5):550-8.
[138] López-Otín et al. 2013.
[139] Chondrogianni, N. and E.S. Gonos 2008. Proteasome activation as a novel antiaging strategy. IUBMB Life 60(10):651-5.
[140] Kruegel, U. et al. 2011. Elevated proteasome capacity extends replicative lifespan in Saccharomyces cerevisiae. PLoS Genetics 7(9):e1002253.
[141] Liang, X.H. et al. 1999. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402:672-6
[142] Kimmelmann, A.C. and E. White 2017. Autophagy and tumor metabolism. Cell Metabolism 25(5): 1037-43.
[143] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5604466/
[144] Ibid.
[145] Alirezaei, M. et al. 2010. Short-term fasting induces profound neuronal autophagy. Autophagy 6(6):702-10.
[146] Chung, K.W. and H.Y. Chung 2019. The effects of calorie restriction on autophagy: Role on aging intervention. Nutrients 11(12):2923.
[147] He, C. et al. 2012. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy 8(10): 1548-51.
[148] Eisenberg, T. et al. 2009. Induction of autophagy by spermidine promotes longevity. Nature Cell Biology 11:1305-14.
[149] https://www.lifebiosciences.com/our-science
[150] https://www.samsaratherapeutics.com/
[151] Carmona-Gutierrez, D. et al. 2019. The flavonoid 4,4′-dimethoxychalcone promotes autophagy-dependent longevity across species. Nature Communications 10, article number: 651.
[152] https://www.casmatx.com/
[153] https://www.neuropore.com/index.htm
[154] https://www.phoremost.com/